
German physicist Max Planck (1858-1947) was born in a traditional, intellectual family. Religion played a big part in the Planck household as both his great grandfather and grandfather were theology professors.
In 1867, Planck was enrolled in the Maximilians gymnasium school, where he came under the guidance of Hermann Müller, a mathematician who immediately recognized Planck's genius.
It was from Müller that Planck first learned the principle of conservation of energy as a 10 year old - that energy can neither be created nor destroyed. This is how Planck first came in contact with the field of physics.
Planck's big problem
When Planck expressed desire to pursue a career in physics, a professor Philipp von Jolly advised him against it, saying: "In physics, almost everything is already discovered."
Planck did not intend to make a discovery of new kind... he simply wanted to study physics deeply. In 1877, aged 19, Planck came under the mentorship of such renowned German scientists as Hermann von Helmholtz and Gustav Kirchhoff.
 |
| Max Planck, 1878 |
Planck was a devoted student with a knack for solving problems. By 1880, he had earned two of the highest degrees offered in Europe - a PhD degree and a qualification for professorship in Universities.
In 1894, Planck started working on the problem of black body radiation as classical theory of light had failed to explain what all was happening.
What is a black body?
A hypothetical black body can absorb all the energy that comes in contact with it, and then because of the laws of thermodynamics, this ideal body must also re-emit as much light as it absorbs.
Spectrum of a near perfect black body at an arbitrary constant temperature is shown below:

All objects actually emit radiation if their temperature is greater than absolute zero. An iron horseshoe, a ceramic cup and even people. The blackbody spectrum tells what is the peak wavelength emitted by that object at that temperature.
Very hot objects will glow - like Tungsten filament in a light bulb at 3300 Kelvin. Human body would emit invisible infrared radiation at 310 Kelvin.
It is important to note that all black body distributions look alike, except that they "peak" in different wavelength regions of the electromagnetic spectrum.
Classical VS quantum
In 1893, Wilhelm Wien had introduced Wien's law, which correctly predicted the behavior of black body at high frequencies - smaller wavelengths, but failed at low frequencies.
The Rayleigh–Jeans law of 1900 agreed with experimental results at low frequencies (below 100 THz), but created an "ultraviolet catastrophe" at higher frequencies.
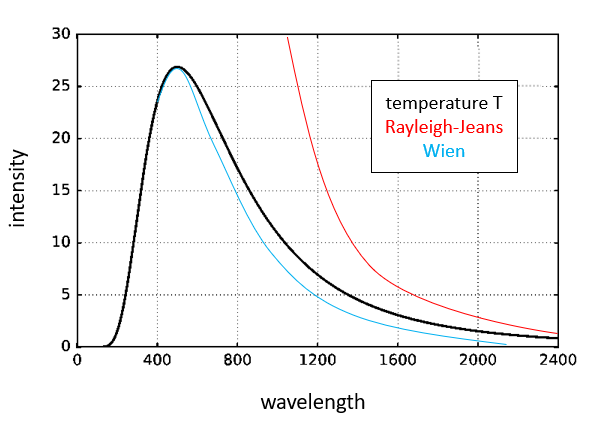
There was no single law or theory that agreed with experimental data at all the values of frequency. Planck was determined to find a solution and at the turn of the century - he did.
In 1901, by assuming that radiation cannot be emitted continuously, as taught by classical physics, but in discrete packets or quanta.
Thus, energy is quantized according to Planck's law.
Planck considered quantization as being purely a mathematical trick and didn't really believe it to be anything more - it just fit the data at hand. In Planck's own words, energy quantum was "purely a formal assumption".
After all, physics is not really about "why" something is true but more about "how" does it work part. Ultimately, by moving away from classical theory Planck was able to explain the shape of black body spectrum to a high degree of accuracy.
Few years later, when Einstein solved another phenomenon where classical theory failed - the photoelectric effect - he gave physical meaning to Planck's energy quantum. The term "photon" was coined and a whole new quantum revolution began.






 Physics, astronomy and science history blog for students
Physics, astronomy and science history blog for students
Responsive Ad Slot