Where did all the chemical elements in the periodic table come from? Physicists theorized that elements can be created inside dying stars and astronomers confirmed this by observation.
Essential elements like carbon, nitrogen, oxygen and iron are created towards the end of a star’s life cycle. Much heavier and precious elements like Gold are formed in supernova explosions.
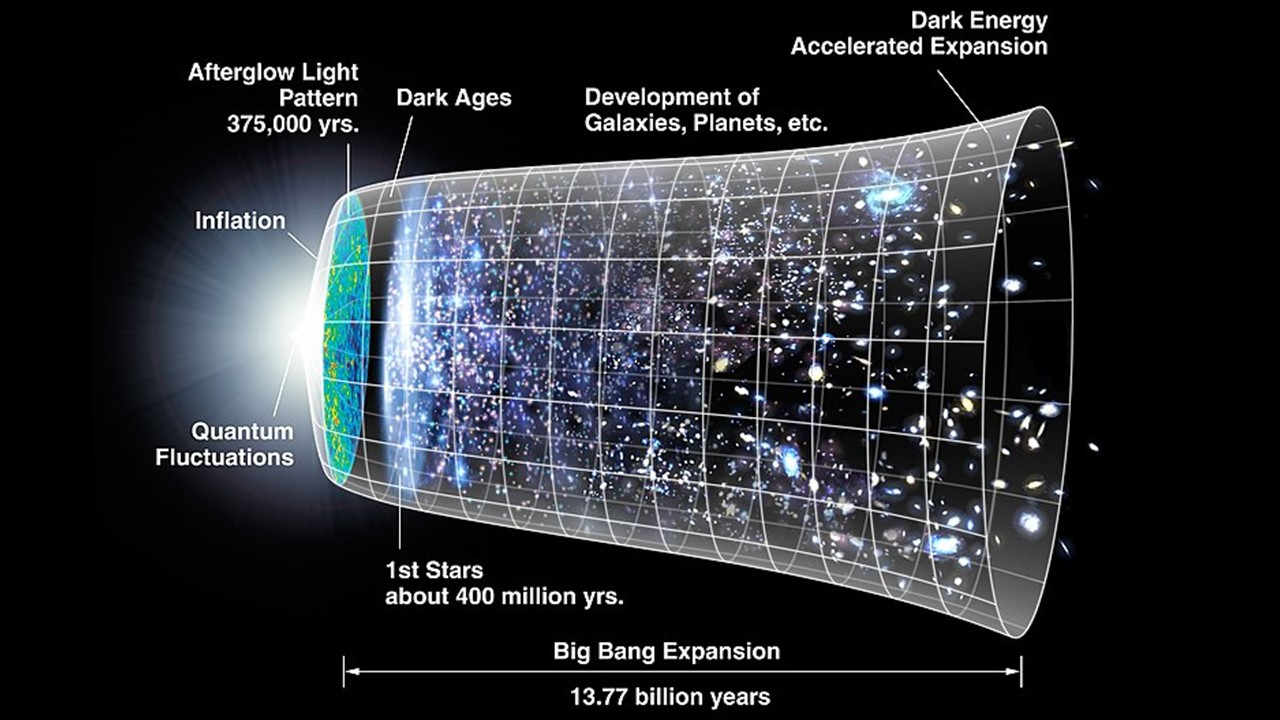
Shortly after the big bang, the explosion that birthed the universe, there was 92% hydrogen and 8% helium atoms. Simple elements came into existence quick, obvious, but what about the rest of them?
Why did nature have to wait for early stars' death in hundreds of millions of years time to produce carbon, oxygen, etc.?
Two reasons: One, by the time simple atoms formed, the universe had already cooled enough. Second, there was hardly any disposable helium.
We know, hydrogen has 1 nucleon, a proton, and helium has 2 protons and 2 neutrons, so 4 nucleons. The reactions to yield heavier elements would be:
H + He or He + He
Giving out nuclei with 5 and 8 nucleons respectively, both highly unstable.
For example: The resulting beryllium-8 has half life of only 8.19×10−17 seconds. Stable beryllium has 5 neutrons and 4 protons.
Thus, beryllium-8 would immediately decay into two stable helium nuclei, if ever it came into being.
Besides, hydrogen and helium are themselves incredibly stable. It turns out that nature preferred stability over creation of heavy elements.
Soon, gigantic lumps of hydrogen began forming due to sophisticated engineering by gravity. The lumps were spherical, because again… nature likes stability.
The first stars made light in extreme conditions upon converting hydrogen to helium, because of Einstein's energy mass equivalence.

Towards the end, most hydrogen in the star is converted to helium. There is abundance of helium nuclei to combine with beryllium-8 in just the right time to become carbon-12.
Ultimately, it boils down to the amount of disposable helium, even if the pressure and temperature conditions are met. The collapsing star makes more elements like nitrogen, oxygen, iron and nickel as it dies.
In the big bang, helium was unavailable for extensive use. Whereas, in the star, formation of carbon is possible in the triple alpha process. And since life on earth is carbon based, we are children of the stars.






 Physics, astronomy and science history blog for students
Physics, astronomy and science history blog for students
Responsive Ad Slot